The short answer is yes, the soap is still natural.
Soap making requires sodium hydroxide, which is more commonly known as caustic soda or lye. That means every soap found in the market have been made using lye. As long as the other ingredients are natural, the soap will stay natural during the soap making process because lye is not present in the final product.
Most soap companies, unlike Men's Soap Company (MSC), leave out the use of lye in their ingredients list because of misinformation and fear among consumers. While lye is dangerous to handle in its chemical form, the substance is not considered as an active ingredient in soaps. Lye is neutralized in the process of saponification, the conversion of fat or oil into soap when combined together.
We at MSC decided to include sodium hydroxide in our ingredients list because you deserve to know how your soap was made. Our belief is that helping you understand this substance is our responsibility so you can feel safe and be confident with your purchase decision.
What Is Sodium Hydroxide, Caustic Soda or Lye?
Sodium Hydroxide is an inorganic compound with the formula NaOH, where Na+ is sodium cations and OH- hydroxide anions. It is a colorless liquid, denser than water, and a highly caustic substance that is used to neutralize acids. Toxicity depends on the concentration of the solution and the duration of its contact with tissue. As a consumer, you will never come in contact with it unless you buy the lye solution for your own product manufacturing process.
Sodium Hydroxide is used in manufacturing a number of products and process you already use or benefit from today:
- Medicines such as aspirin, anticoagulants, and cholesterol-reducers
- Water treatment process to control water acidity and remove heavy metals
- Paper recycling process
- Food and vegetable deskinning and canning process
- Cleaning and disinfectant products
The Science of Saponification
Saponification is the chemical reaction when fat or oil is mixed with an alkali such as sodium hydroxide, the result of this reaction being soap and glycerin. Or, as UCLA Illustrated Glossary of Organic Chemistry puts it, "The process in which a triacylglyceride is reacted with aqueous hydroxide ion to form a mixture of glycerol and fatty acid salts (soaps). The reaction mechanism follows the nucleophilic carbonyl substitution pathway."
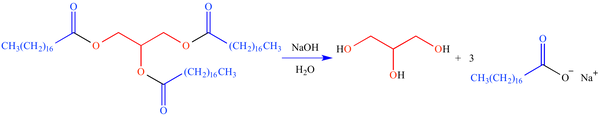
Saponification of glycerol tristearate (a triacylglyceride) with NaOH (lye) yields a soap consisting of one part glycerol (shown in red) and three parts sodium stearate (shown in blue).
As you can see, there is no sodium hydroxide or lye in the finished product, only soap.
Natural Hand Made Soap in Small Batches
If you're curious about making natural soap yourself at home, it's not a difficult process but it can be dangerous. For this obvious and legal reasons, we cannot guide you through the soap making process. We encourage you to do thorough research on the proper handling of lye and the safety measures pertinent to soap making.
A basic soap recipe just needs a few essential ingredients: Water (distilled or spring), vegetable fat, essential oils for fragrance, and lye (sodium hydroxide) of course. However, you may want to consider an additional list of ingredients such as coconut oil and shea butter that are beneficial to the skin.
Lye for soap making is easier to get in a small amount, and they are usually sold in the form of flakes. Obtaining a large quantity for a soap business may be a bit trickier, as shipping of this substance is tightly controlled and regulated. We recommend making soaps in small batches so you can control and test your batches for quality, especially when the process is completed mostly by hand and hand tools.
There are plenty of soap making recipes and instructions online. When in doubt, Google is your best friend. Be careful and have fun!
